Regulatory Affairs in Pharmaceutical Industry
Pharmaceutical and biotechnology regulatory affairs is a complex and ever-changing field, where non-compliance and non-compliance with requirements can result in severe penalties, fines and reputational damage.
This poses many challenges to the pharmaceutical industry, which can be described in the different stages of the healthcare product life cycle:
- Regulatory planning at the development stage
- Proof of regulatory requirements for approval
- Post-approval vigilance
Strategic regulatory planning at the development stage
Understanding the intricacies of applicable regulations and adopting a nuanced approach that takes into account the needs of different stakeholders while recognizing the inherent risks are critical to the success of any business. Moreover, by staying ahead of the curve through proper planning, companies ensure that they meet all requirements, putting them in a better position for future success.
As a result, regulatory affairs professions are playing an increasingly strategic and cross-functional role within the company.
In addition to contributing to the applicability and demonstration of current requirements, they act as advisors and pillars for the rest of the company throughout the life cycle of healthcare products, from molecule to finished product.
Evidence of regulatory requirements for approval
Obtaining approval from the relevant health authorities is one of the most difficult aspects of regulatory affairs: providing proof of compliance with the requirements in force by drafting the regulatory file, called the CTD (Common Technical Document). This file, organized into five modules, contains detailed documentation of the development and manufacturing process of the health product. It is essential to demonstrate the quality, safety and efficacy of the proposed product.
To satisfy the regulatory authorities, every detail must be specified and, if necessary, pre-clinical and clinical trials must be conducted accordingly. This process can be long and costly and requires careful attention to detail throughout the application process.
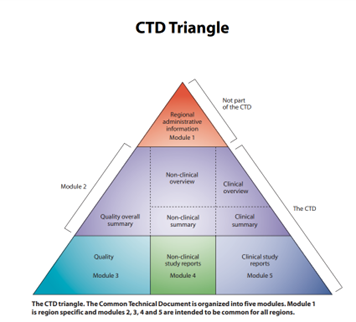
Figure 1: CTD Triangle (Source: ICH Standards – CTD)
Pre-market approval requirements differ by region, making it essential for companies to know the countries where their products will be sold as early as possible in the development of the health product. This requires constant monitoring of the relevant legislation in force and the guidelines applicable to the countries of operation. Similarly, manufacturers must ensure that they comply with local Good Manufacturing Practice (GMP) guidelines when producing their products.
Post-Approval Vigilance
In addition to approving healthcare products for marketing, another key challenge is staying compliant with changing legislation and industry standards. This includes keeping abreast of new laws introduced both domestically and internationally, in all markets where they operate, as well as being vigilant about changes to existing regulations so that they can proactively update their policies and procedures accordingly.
Another mission of regulatory affairs is pharmacovigilance, the purpose of which is to monitor health products on the market and prevent the risk of potential or proven adverse effects related to their use. It is essential because it constitutes a guarantee that is exercised throughout the life of a health product.
Ultimately, the management of regulatory affairs issues for pharmaceuticals and biotechnology products requires a scientific and technical understanding of the end product, its use and manufacture, ingenuity in implementing applicable standards and a willingness to take calculated risks.
By doing so, your organization will maximize the chances of remaining in compliance and avoiding costly penalties, ensuring its long-term viability for years to come.



